Download the latest research brochure - Ben-Yakar-FemtoLAB.pdf (396KB)
|
|
Nerve regeneration studies in Caenorhabditis elegans using laser axotomy and RNA interference |
 |
 |
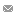 |
|
Current Research
|
|
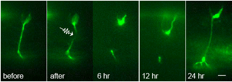 
Top: Nerve regeneration after femtosecond laser axotomy in live C. elegans. Fluorescence images of GFP labeled axons before, after, and following axotomy. Bottom: Rendition of a trapped worm undergoing axotomy in a microfluidic chip.
|
In the United States, nearly 5 million people suffer from neurodegenerative diseases and another half?million have suffered from some type of nerve injury. To this day, the mechanisms underlying nerve regeneration and degeneration remain largely unknown. Searching for the genes involved in these mechanisms will help us to better understand the causes of these debilitating diseases and thus pave the way for successful treatments and preventions. The best conditions for studying nerve regeneration would be met if one could sever axons or dendrites in a controlled manner and study what genes or molecules affect their regrowth in vivo in a simple organism. The soil nematode Caenorhabditis elegans (C. elegans) is an ideal model organism for such study. They are transparent to visible light; their nervous system comprises only 302 neurons and is entirely mapped; and their genetic versatility allows for gene manipulations. In our animal model, C. elegans, we are investigating mutant worms and using RNA interference, along with femtosecond laser surgery, to search for candidate genes that may be involved in this complicated regeneration process.
|
|
|
|
Microfluidics for high-throughput nerve regeneration studies in Caenorhabditis elegans |
 |
 |
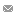 |
|
Current Research
|
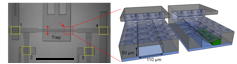 
Top left: Magnified view of the trapping system. Scale bar: 1 mm. Top right: Conceptual 3D sectional renderings of the bilayer trap channels without and with an immobilized worm. Bottom: Rendition of the multiplexor?interfaced well?plate.
|
Nerve regeneration studies should utilize an environment for both surgery and observation that has minimal impacts on biological pathways and behavior. In contrast to the conventional immobilization method using anesthetics, microfluidic devices offer several advantages, including chemical?free immobilization, ease of manipulation, and high throughput data acquisition. These devices provide a wellcontrolled microenvironment for severing axons and monitoring the animals’ neural functions. Specifically for nerve regeneration studies, we developed a new microfluidic device to immobilize the worms for surgery without using any chemicals. This microfluidic device allows us to precisely manipulate worms inside small channels and eliminates the need for anesthetics, which we have shown to interfere with the axonal regrowth process. With the recent advancements in microfluidic interfaces, now it is also feasible to automatically load large sample populations from multiwell plates. The prospective integration and full automation of such microfluidic platforms finally offers the possibility to perform high?throughput, genome?wide screening of phenotypes in living animals.
|
|
|
Clinical endoscope for two-photon image-guided femtosecond laser surgery |
 |
 |
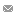 |
|
Current Research
|

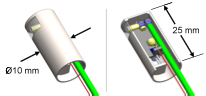
Left: Photograph of the first probe prototype, measuring just 10 × 15 × 40 mm3. Center: A two-photon fluorescence image of cancer cells taken with the prototype, indicating a cell targeted for ablation. Right: The same region, after delivering one high-energy surgery pulse to the targeted cell. The targeted cell was instantly destroyed while neighboring cells were left intact. Below: A preliminary model of a clinically packaged endoscope for treatment of vocal fold scars.
|
Two-photon microscopy is emerging as a useful imaging technique for diagnostic imaging of biological tissues. Additionally, femtosecond laser pulses have proven to be much more efficient in ablating tissue for microsurgery, thus greatly reducing collateral damage. Thus far, integration of these techniques into clinically-useful tools has been extremely limited. The aim of this project is to develop small flexible probes capable of delivering ultrashort laser pulses into the body for combined imaging and microsurgery. Currently, we are focused on two key clinical applications: treatment of small cancerous lesions and treatment of vocal fold scarring. This project has strong optical design and experimental components. The nature of the work is very multidisciplinary and includes fields such as MEMS devices, photonic crystal structures, manufacturing, signal processing, and cell biology. There are currently several proposals being reviewed to provide funding for this project. |
|
|
Plasmonic Laser Nanosurgery (PLN) |
 |
 |
 |
|
Current Research
|
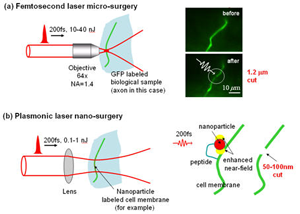 |
Plasmonic Laser Nanosurgery (PLN) is a novel photodisruption technique that exploits the large enhancement of femtosecond (fs) laser pulses in the near?field of metal nanoparticles for the selective and non?thermal nanoscale manipulation of biological structures. The electric field, which is amplified when the laser frequency is tuned to the plasmon frequency of a metal nanostructure, can be utilized to initiate photodamage with nanoscale precision to biological targets onto which nanoparticles are attached. The use of fs?laser pulses ensures non?thermal tissue photodisruption, while functionalized nanoparticles greatly improve the localization of the photodisruption process. Moreover, the enhanced electric field around the particle reduces the fluence necessary for material photodisruption, limiting the extent of damage to the particle near?field. Since the particles themselves act as “nano?lenses”, large volumes of tissue can be irradiated at a given time, decreasing the time of treatment. In addition, gold nanostructures exhibit minimal cellular toxicity, making the technique ideal for in vivo clinical use. .
In our own research, we have demonstrated the feasibility of plasmonic laser optoporation for both cellular death by necrosis for cancer treatment and transient pore formation for cellular transfection. In clinical medicine, PLN has the potential to treat cancers localized in sensitive regions, i.e. brain tumor, or can be utilized for the removal of small epithelial cancer lesions, i.e. breast carcinomas in situ. In biological sciences, PLN could bring significant advance in the transfection of cellular systems; this includes the transfection of cells that typically do not respond well to traditional transfection methods or that of in vivo mass transfections. On a broader spectrum, the high specificity of PLN provides the possibility for membrane? and molecular?specific phototherapy; direct therapeutic prospects extend into the fields of cancer, genetics, proteomics, virology, bacteriology, and cardiology.
Specific project: Demonstrate laser surgery at the nano-scale (about 20-50nm).
|
|
|
|
<< Start < Prev 1 2 Next > End >>
|
|
Page 1 of 2 |




