|
Microfluidics for high-throughput nerve regeneration studies in Caenorhabditis elegans |
 |
|
Current Research
|
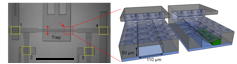 
Top left: Magnified view of the trapping system. Scale bar: 1 mm. Top right: Conceptual 3D sectional renderings of the bilayer trap channels without and with an immobilized worm. Bottom: Rendition of the multiplexor?interfaced well?plate.
|
Nerve regeneration studies should utilize an environment for both surgery and observation that has minimal impacts on biological pathways and behavior. In contrast to the conventional immobilization method using anesthetics, microfluidic devices offer several advantages, including chemical?free immobilization, ease of manipulation, and high throughput data acquisition. These devices provide a wellcontrolled microenvironment for severing axons and monitoring the animals’ neural functions. Specifically for nerve regeneration studies, we developed a new microfluidic device to immobilize the worms for surgery without using any chemicals. This microfluidic device allows us to precisely manipulate worms inside small channels and eliminates the need for anesthetics, which we have shown to interfere with the axonal regrowth process. With the recent advancements in microfluidic interfaces, now it is also feasible to automatically load large sample populations from multiwell plates. The prospective integration and full automation of such microfluidic platforms finally offers the possibility to perform high?throughput, genome?wide screening of phenotypes in living animals.
|
|

